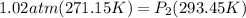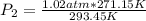Chemistry, 24.06.2019 16:00 sofiisabella10
Sample of atmospheric gas collected at an industrial site is stored in a 250 ml amber glass bottle that has a pressure of 1.02 atm and a temperature of 20.3°c. it was placed in an ice chest whose internal temperature is -2.0°c. what will be the new pressure of the gas sample once the gas temperature in the jar equilibrates to that of the ice chest?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 09:00
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 23.06.2019 01:00
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
Sample of atmospheric gas collected at an industrial site is stored in a 250 ml amber glass bottle t...
Questions


English, 25.05.2021 04:00



Mathematics, 25.05.2021 04:00

Mathematics, 25.05.2021 04:00

French, 25.05.2021 04:00

Physics, 25.05.2021 04:00


Mathematics, 25.05.2021 04:00

English, 25.05.2021 04:00





History, 25.05.2021 04:00


Mathematics, 25.05.2021 04:00

History, 25.05.2021 04:00

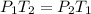
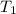 and
and 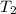 are initial and final temperatures,
are initial and final temperatures, 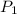 and
and 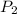 are initial and final pressures.
are initial and final pressures.