
Chemistry, 24.06.2019 13:00 natalia9573
How many grams of oxygen are required to react with 20.0 grams of octane in the combustion of octane in gasoline?
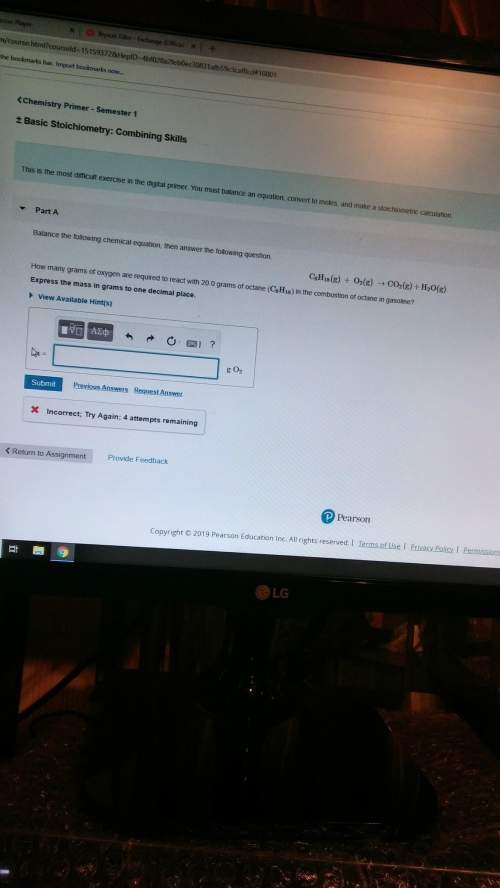

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 22.06.2019 19:00
Imagine that a new planet is discovered with two moons of equal mass: moon a and moon b. the mass of the new planet is greater than the combined mass of its moons. moon a is farther away from the new planet than moon b. what is the planet's gravitational pull on moon a compared to the planet's gravitational pull on moon b? the planet's gravity repels moon a with a greater force than it repels moon b, which is why moon a is farther away. the gravitational pull on moon b is greater than on moon a because moon b is closer to the new planet than moon a. the gravitational pull on moon b is greater than on moon a because moon b is farther away from the new planet than moon a. the gravitational pull on moon a is the same as the gravitational pull on moon b because distance does not affect the planet's gravity.
Answers: 1

Chemistry, 22.06.2019 20:40
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1

Chemistry, 22.06.2019 23:30
Why do oxygen have a strong attractive force for electrons
Answers: 2
You know the right answer?
How many grams of oxygen are required to react with 20.0 grams of octane in the combustion of octane...
Questions


Geography, 01.06.2020 17:57

English, 01.06.2020 17:57

Computers and Technology, 01.06.2020 17:57

Mathematics, 01.06.2020 17:57

Social Studies, 01.06.2020 17:57



History, 01.06.2020 17:57



Mathematics, 01.06.2020 17:57

Social Studies, 01.06.2020 17:57

Computers and Technology, 01.06.2020 17:57


Biology, 01.06.2020 17:57

Mathematics, 01.06.2020 17:57

Mathematics, 01.06.2020 17:57

Mathematics, 01.06.2020 17:57



