
Chemistry, 30.11.2019 08:31 SuperWoman9172
Carbon disulfide burns in oxygen to yield car- bon dioxide and sulfur dioxide according to the chemical equation cs2(l) 3 o2(g) −→ co2(g) 2 so2(g). if 0.91 mol of cs2 is combined with 1.52 mol of o2, identify the limiting reactant.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2



Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3
You know the right answer?
Carbon disulfide burns in oxygen to yield car- bon dioxide and sulfur dioxide according to the chemi...
Questions

Mathematics, 07.12.2020 20:00

Mathematics, 07.12.2020 20:00

English, 07.12.2020 20:00

Mathematics, 07.12.2020 20:00

Mathematics, 07.12.2020 20:00


Mathematics, 07.12.2020 20:00







Mathematics, 07.12.2020 20:00

Biology, 07.12.2020 20:00

Mathematics, 07.12.2020 20:00

Social Studies, 07.12.2020 20:00



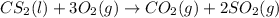
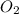 reacts with 1 mole of
reacts with 1 mole of 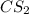
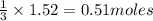 of
of 

