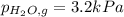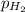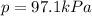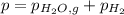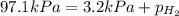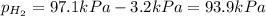When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle. if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3.2 kilopascals, what is the partial pressure of the hydrogen?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted to br2(g). s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 3

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1

Chemistry, 23.06.2019 02:00
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2

Chemistry, 23.06.2019 09:40
Write balanced nuclear equations for the formation of five elements whose atomic number is between helium (2) and iron (26):
Answers: 1
You know the right answer?
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water va...
Questions


Mathematics, 19.02.2021 01:00


Arts, 19.02.2021 01:00


Mathematics, 19.02.2021 01:00

World Languages, 19.02.2021 01:00

Mathematics, 19.02.2021 01:00

Geography, 19.02.2021 01:00

Mathematics, 19.02.2021 01:00

Mathematics, 19.02.2021 01:00

Mathematics, 19.02.2021 01:00

Social Studies, 19.02.2021 01:00

Mathematics, 19.02.2021 01:00



Computers and Technology, 19.02.2021 01:00


Chemistry, 19.02.2021 01:00

Mathematics, 19.02.2021 01:00