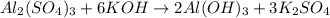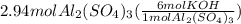Chemistry, 23.06.2019 09:00 hunterwilliams375
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate according to the following equation:
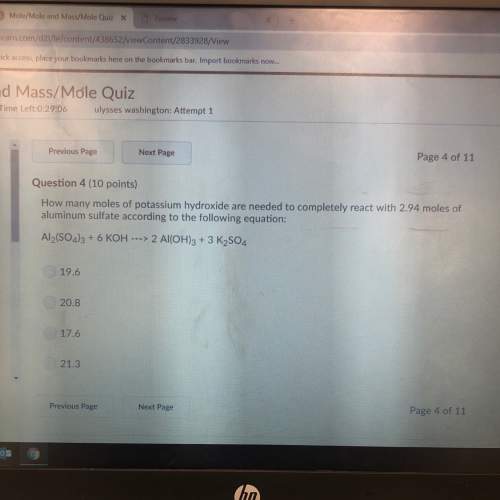

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3
You know the right answer?
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sul...
Questions


Mathematics, 24.04.2020 06:44

Mathematics, 24.04.2020 06:44

Mathematics, 24.04.2020 06:44


Mathematics, 24.04.2020 06:44




Chemistry, 24.04.2020 06:44


Mathematics, 24.04.2020 06:44

History, 24.04.2020 06:44


Chemistry, 24.04.2020 06:44

History, 24.04.2020 06:44

Mathematics, 24.04.2020 06:44


Geography, 24.04.2020 06:44

Mathematics, 24.04.2020 06:44