
Chemistry, 22.06.2019 23:30 Xavier8247
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 18:00
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3

You know the right answer?
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , c...
Questions


Chemistry, 22.01.2021 20:20



Mathematics, 22.01.2021 20:20



Mathematics, 22.01.2021 20:20

Chemistry, 22.01.2021 20:20


Spanish, 22.01.2021 20:20

Mathematics, 22.01.2021 20:20

Mathematics, 22.01.2021 20:20


History, 22.01.2021 20:20

Chemistry, 22.01.2021 20:20

Geography, 22.01.2021 20:20

English, 22.01.2021 20:20


Mathematics, 22.01.2021 20:20

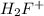 will not give proton easily. Therefore, its acidity will be the least.
will not give proton easily. Therefore, its acidity will be the least.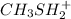 is slightly more than
is slightly more than 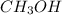 will have highest bronsted acidity and
will have highest bronsted acidity and 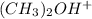 will be less acidic than
will be less acidic than 

