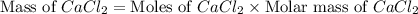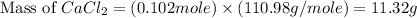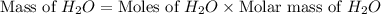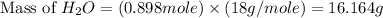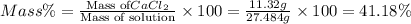Chemistry, 09.01.2020 00:31 nehakarakkattu
"an aqueous cacl2 solution has a vapor pressure of 83.1mmhg at 50 ∘c. the vapor pressure of pure water at this temperature is 92.6 mmhg. what is the concentration of cacl2 in mass percent? "

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

You know the right answer?
"an aqueous cacl2 solution has a vapor pressure of 83.1mmhg at 50 ∘c. the vapor pressure of pure wat...
Questions


Mathematics, 07.01.2021 22:20

Mathematics, 07.01.2021 22:20

Mathematics, 07.01.2021 22:20


Health, 07.01.2021 22:20

Mathematics, 07.01.2021 22:20

English, 07.01.2021 22:20




Mathematics, 07.01.2021 22:20


Mathematics, 07.01.2021 22:20



Mathematics, 07.01.2021 22:20


History, 07.01.2021 22:20

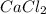 in mass percent is, 41.18 %
in mass percent is, 41.18 %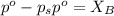
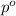 = vapor pressure of the pure component (water) = 92.6 mmHg
= vapor pressure of the pure component (water) = 92.6 mmHg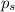 = vapor pressure of the solution = 83.1 mmHg
= vapor pressure of the solution = 83.1 mmHg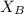 = mole fraction of solute,
= mole fraction of solute, 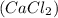
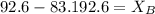
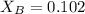
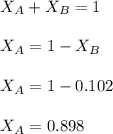
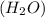 .
.