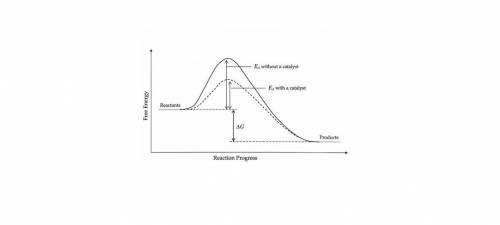
Which of the following phase diagrams represents how a catalyst is able to change the rate of a reaction? (2 points) a regular exothermic potential energy diagram is shown, with a dotted line representing how the potential energy diagram changes with the use of a catalyst. the dotted line shows a new potential energy diagram with two potential energy hills, instead of the one hill in the regular potential energy diagram. a regular exothermic potential energy diagram is shown, with a dotted line representing how the potential energy diagram changes with the use of a catalyst. the dotted line shows a new potential energy diagram with a taller activation energy hill than that in the original potential energy diagram. a regular exothermic potential energy diagram is shown, with a dotted line representing how the potential energy diagram changes with the use of a catalyst. the dotted line shows a new potential energy diagram with two activation energy hills, the second taller than the first, instead of the one hill in the regular potential energy diagram. a regular exothermic potential energy diagram is shown, with a dotted line representing how the potential energy diagram changes with the use of a catalyst. the dotted line shows a new potential energy diagram with a shorter activation energy hill than that in the original potential energy diagram.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:10
How does chemistry affect our world? a. chemicals makes our world more polluted. b. chemicals keeps us healthy. c. chemicals can or hurt our world. d. chemicals make our world safe to live in.
Answers: 1

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1

Chemistry, 22.06.2019 13:00
Adepositional also feature that forms where a stream enters a lake or an ocean is a
Answers: 2
You know the right answer?
Which of the following phase diagrams represents how a catalyst is able to change the rate of a reac...
Questions


Biology, 19.07.2019 05:50




Mathematics, 19.07.2019 05:50


History, 19.07.2019 05:50


Mathematics, 19.07.2019 05:50


Social Studies, 19.07.2019 05:50

Mathematics, 19.07.2019 05:50




Mathematics, 19.07.2019 05:50



Mathematics, 19.07.2019 05:50