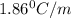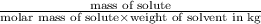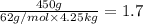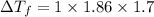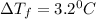Ethylene glycol (molar mass = 62 g/mol)is used as an antifreeze in cars. if 450 g of ethylene glycol is added to 4.25 kg of water, what is the molality? calculate how much the freezing point of water will be lowered. the freezing point depression constant for water is kf = -1.86°c/m. show your work.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3

Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

You know the right answer?
Ethylene glycol (molar mass = 62 g/mol)is used as an antifreeze in cars. if 450 g of ethylene glycol...
Questions

Social Studies, 28.02.2021 15:10


Medicine, 28.02.2021 15:10


English, 28.02.2021 15:10


Mathematics, 28.02.2021 15:10


Mathematics, 28.02.2021 15:10

English, 28.02.2021 15:10



Mathematics, 28.02.2021 15:10



Arts, 28.02.2021 15:20

Mathematics, 28.02.2021 15:20


English, 28.02.2021 15:20

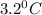
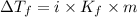
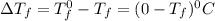 = Depression in freezing point
= Depression in freezing point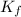 = freezing point constant =
= freezing point constant =