
Chemistry, 22.01.2020 02:31 alexjedington
iron (iii) oxide reacts with solid carbon in the followed reaction: 2fe2o3(s) + 3c(s) → 4fe(s) + 3co2(g) what mass of fe2o3 is necessary to produce 100. l of co2 at 300. k and 2.10 atm? 823 g fe2o3 908 g fe2o3 1,110 g fe2o3 1,360 g fe2o3

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Arock can be broken down into different kinds of substances by physical processes. no chemical reactions are needed to separate different parts of a rock into pure substances. this is because a rock is a(n)
Answers: 1


Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 23:00
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3
You know the right answer?
iron (iii) oxide reacts with solid carbon in the followed reaction: 2fe2o3(s) + 3c(s) → 4fe(s) + 3c...
Questions

Social Studies, 02.12.2020 23:40



Biology, 02.12.2020 23:40

Mathematics, 02.12.2020 23:40

Biology, 02.12.2020 23:40

Mathematics, 02.12.2020 23:40


Biology, 02.12.2020 23:40

Social Studies, 02.12.2020 23:40



English, 02.12.2020 23:40

Mathematics, 02.12.2020 23:40


Physics, 02.12.2020 23:40




History, 02.12.2020 23:40

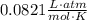 T is temperature (in Kelvins)
T is temperature (in Kelvins)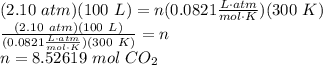
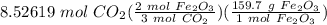 = 907.755 g Fe₂O₃
= 907.755 g Fe₂O₃

