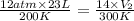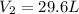Chemistry, 17.10.2019 15:20 naiquawhite
If i initially have a gas at a pressure of 12 atm, volume of 23 liters, and temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3
You know the right answer?
If i initially have a gas at a pressure of 12 atm, volume of 23 liters, and temperature of 200 k, an...
Questions













Mathematics, 18.12.2020 17:00



Mathematics, 18.12.2020 17:00

Biology, 18.12.2020 17:00



Mathematics, 18.12.2020 17:00

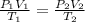
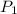 = initial pressure of gas = 12 atm
= initial pressure of gas = 12 atm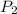 = final pressure of gas = 14 atm
= final pressure of gas = 14 atm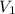 = initial volume of gas = 23 L
= initial volume of gas = 23 L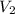 = final volume of gas = ?
= final volume of gas = ?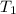 = initial temperature of gas = 200K
= initial temperature of gas = 200K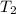 = final temperature of gas = 300K
= final temperature of gas = 300K