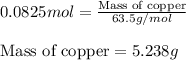Chemistry, 08.10.2019 23:30 weirdoal567
Achemist wants to extract copper metal from copper chloride solution. the chemist places 1.50 grams of aluminum foil in a solution of 14 grams of copper (ii) chloride. (single replacement reaction.) what best explains the state of the reaction mixture afterward?
a. less than 6.0 grams of copper (ii) is formed, and some aluminum is left in the reaction mixture.
b. more than 6.5 grams of copper (ii) is formed, and some aluminum is left in the reaction mixture.
c. less than 6.0 grams of copper (ii) is formed, and some copper chloride is left in the reaction mixture.
d. more than 6.5 grams of copper (ii) is formed, and some copper chloride is left in the reaction mixture.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What is the empirical formula of vanadium 1 oxide given that 20.38 grams of vandium combines with oxygen to form 23.58 grams of the oxide
Answers: 1

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 22:00
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2

Chemistry, 23.06.2019 05:30
Idont understand it 1.what is the boiling point of a solution of 675 grams of ethylene glycol (c2h6o2) in 2.50 liters of water?
Answers: 2
You know the right answer?
Achemist wants to extract copper metal from copper chloride solution. the chemist places 1.50 grams...
Questions


Social Studies, 27.10.2020 03:20


Biology, 27.10.2020 03:20

Mathematics, 27.10.2020 03:20



Mathematics, 27.10.2020 03:20

Mathematics, 27.10.2020 03:20

History, 27.10.2020 03:20


Biology, 27.10.2020 03:20

Mathematics, 27.10.2020 03:20



English, 27.10.2020 03:20



Mathematics, 27.10.2020 03:20

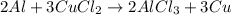
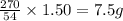 of copper chloride.
of copper chloride.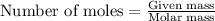 ....(1)
....(1)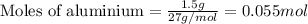
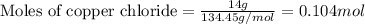
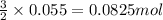 of copper chloride.
of copper chloride.