
Chemistry, 03.12.2019 02:31 carlydays3331
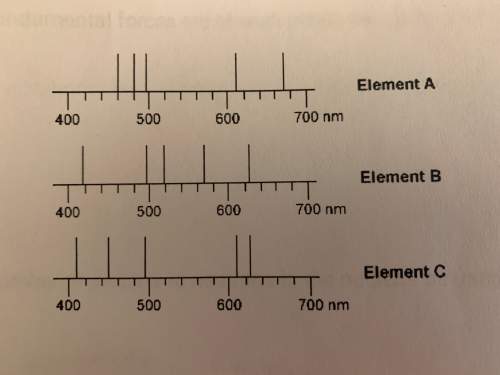
Ahigh school student acquired an emission spectrum of an unknown sample. he knew the unknown sample contained one of the elements, whose spectra are show below. the emission spectrum of his sample showed a strong emission at 610 nm but not at 480 nm.
a. which element did his unknown sample contain?
b. element a above has a strong emission around 670nm. does this emission line represent a lower energy or higher energy transition than the emission line at 428nm?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2


Chemistry, 23.06.2019 07:30
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes or no?
Answers: 1
You know the right answer?
Ahigh school student acquired an emission spectrum of an unknown sample. he knew the unknown sample...
Questions

Mathematics, 10.11.2020 18:20

Mathematics, 10.11.2020 18:20

History, 10.11.2020 18:20


Social Studies, 10.11.2020 18:20

Mathematics, 10.11.2020 18:20

Mathematics, 10.11.2020 18:20

English, 10.11.2020 18:20




Mathematics, 10.11.2020 18:20


Mathematics, 10.11.2020 18:20

Mathematics, 10.11.2020 18:20



Computers and Technology, 10.11.2020 18:20

Mathematics, 10.11.2020 18:20



