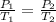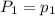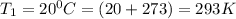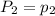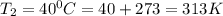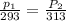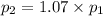Chemistry, 05.02.2020 02:52 alexandroperez13
To understand the ideal gas law and be able to apply it to a wide variety of situations. the absolute temperature t, volume v, and pressure p of a gas sample are related by the ideal gas law, which states that pv=nrt. here n is the number of moles in the gas sample and r is a gas constant that applies to all gases. this empirical law describes gases well only if they are sufficiently dilute and at a sufficiently high temperature that they are not on the verge of condensing. in applying the ideal gas law, p must be the absolute pressure, measured with respect to vacuum and not with respect to atmospheric pressure, and t must be the absolute temperature, measured in kelvins (that is, with respect to absolute zero, defined throughout this tutorial as ^ -273˚c). if p is in pascals and v is in cubic meters, use r=8.3145j/(mol x k). if p is in atmospheres and v is in liters, use r=0.08206l x atm/(mol x k) instead. part a a gas sample enclosed in a rigid metal container at room temperature (20.0˚c) has an absolute pressure p1. the container is immersed in hot water until it warms to 40.0˚c. what is the new absolute pressure p2?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1


Chemistry, 23.06.2019 05:40
Which order shows the levels of organization from largest to smallest? organism, organ system, cell, organ, tissue organism, tissue, organ system, organ, cell organism, organ, organ system, cell, tissue organism, organ system, organ, tissue, cell
Answers: 2
You know the right answer?
To understand the ideal gas law and be able to apply it to a wide variety of situations. the absolut...
Questions

Mathematics, 08.04.2021 23:00


Mathematics, 08.04.2021 23:00

Mathematics, 08.04.2021 23:00

Mathematics, 08.04.2021 23:00



Mathematics, 08.04.2021 23:00

Advanced Placement (AP), 08.04.2021 23:00






Mathematics, 08.04.2021 23:00

History, 08.04.2021 23:00


Mathematics, 08.04.2021 23:00

Mathematics, 08.04.2021 23:00

Geography, 08.04.2021 23:00

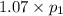
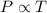 (At constant volume and number of moles)
(At constant volume and number of moles)