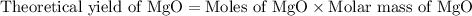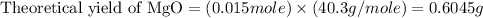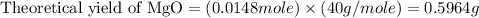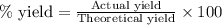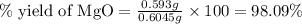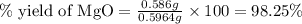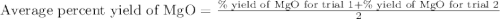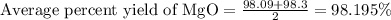Chemistry, 10.11.2019 17:31 shortty1111
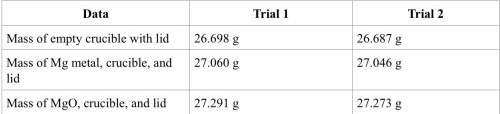
1. calculate the actual yield of magnesium oxide for each trial.
trial 1:
trial 2:
2. magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial.
trial 1:
trial 2:
3. determine the percent yield of mgo for your experiment for each trial.
trial 1:
trial 2:
4. determine the average percent yield of mgo for the two trials.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:50
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3


Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1
You know the right answer?
1. calculate the actual yield of magnesium oxide for each trial.
trial 1:
trial...
trial 1:
trial...
Questions



Social Studies, 26.07.2019 12:10

Chemistry, 26.07.2019 12:10


Computers and Technology, 26.07.2019 12:10

History, 26.07.2019 12:10


Mathematics, 26.07.2019 12:10

Mathematics, 26.07.2019 12:10


English, 26.07.2019 12:10

Mathematics, 26.07.2019 12:10



History, 26.07.2019 12:10


Biology, 26.07.2019 12:10


Chemistry, 26.07.2019 12:10

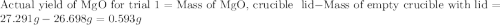
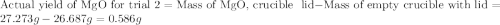
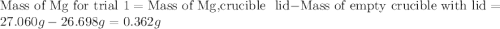
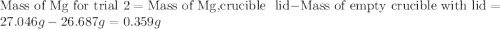
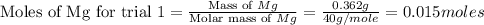
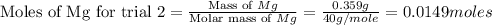
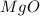 for trial 1 and trial 2.
for trial 1 and trial 2.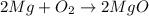
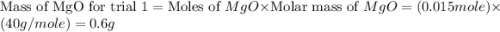
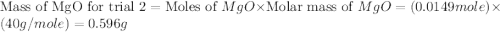
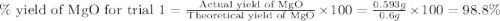
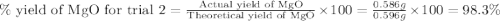

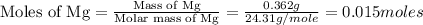
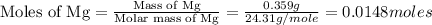
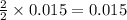 moles of MgO.
moles of MgO.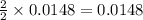 moles of MgO.
moles of MgO.