
Chemistry, 04.02.2020 16:58 genesisdiaz1352
Calculate the number of atoms of carbon (c) in 340. cm3 of the colorless gas methylacetylene at 0 °c and atmospheric pressure, where its density is 1.79×10-3 g cm-3. the molecular formula of methylacetylene is c3h4.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2


You know the right answer?
Calculate the number of atoms of carbon (c) in 340. cm3 of the colorless gas methylacetylene at 0 °c...
Questions




Mathematics, 08.12.2021 21:20

Mathematics, 08.12.2021 21:20



Mathematics, 08.12.2021 21:20


Social Studies, 08.12.2021 21:20



Mathematics, 08.12.2021 21:20







History, 08.12.2021 21:20

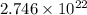
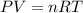
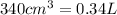 (Conversion Factor:
(Conversion Factor: 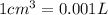 )
)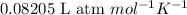
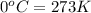 (Conversion factor:
(Conversion factor: 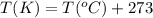 )
)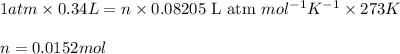
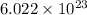 number of atoms
number of atoms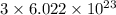 number of C-atoms.
number of C-atoms.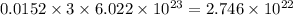 number of C-atoms.
number of C-atoms.

