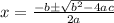Chemistry, 06.10.2019 15:20 nefertiri64
Find the ph of a solution containing 0.025 m chloric acid and 0.35 m hypochlorous acid.
i don’t know if you need the ka value to solve this so i included it anyway.
ka of hclo2 = 1.2x10^-2
ka of hocl = 3.5x10^-8

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1

Chemistry, 23.06.2019 02:30
Which words or phrases identify layers of groundwater? check all that apply. water table kettle lake saturation zone underground lake sinkhole will give brainiest, answer quickly.
Answers: 1
You know the right answer?
Find the ph of a solution containing 0.025 m chloric acid and 0.35 m hypochlorous acid.
...
...
Questions

English, 24.11.2021 20:30

History, 24.11.2021 20:30

Chemistry, 24.11.2021 20:30

French, 24.11.2021 20:30




Business, 24.11.2021 20:40



Mathematics, 24.11.2021 20:40