
Chemistry, 03.02.2020 13:52 terrybrown5391
Consider the reaction alatex: \longrightarrow ⟶ products. the rate law for this reaction is rate = k[a]2 where k=2.90 latex: \times × 10-2 m-1s-1 at a particular temperature. if the initial [a] = 0.0500 m, what is the value of the half-life?
478 s
34.5 s
690. s
23.9 s

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1

Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2

Chemistry, 23.06.2019 00:30
Fred is studying a substance that is made out of only one element. this means that
Answers: 1
You know the right answer?
Consider the reaction alatex: \longrightarrow ⟶ products. the rate law for this reaction is rate =...
Questions

History, 04.02.2020 12:45




History, 04.02.2020 12:45


Mathematics, 04.02.2020 12:45

Mathematics, 04.02.2020 12:45

English, 04.02.2020 12:45

History, 04.02.2020 12:45




Mathematics, 04.02.2020 12:45


Mathematics, 04.02.2020 12:45

Physics, 04.02.2020 12:45


Mathematics, 04.02.2020 12:45

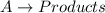
![Rate=k[A]^2](/tpl/images/0496/6747/e6b6e.png)
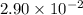
![t_\frac{1}{2}=\frac{1}{k\times [A_0]}](/tpl/images/0496/6747/95401.png)
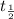 = half life = time taken for a reaction to complete to half.
= half life = time taken for a reaction to complete to half. ![[A_0]](/tpl/images/0496/6747/9a686.png) =initial concentration= 0.0500 M
=initial concentration= 0.0500 M![t_{\frac{1}{2}=\frac{1}{2.90\times 10^{-2}\times [0.0500]}=690s](/tpl/images/0496/6747/bf804.png)


