

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 19:50
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2
You know the right answer?
Consider the reaction below. at 500 k, the reaction is at equilibrium with the following concentrati...
Questions


Mathematics, 23.02.2021 18:10

Mathematics, 23.02.2021 18:10






English, 23.02.2021 18:10


Computers and Technology, 23.02.2021 18:10





Mathematics, 23.02.2021 18:10

Arts, 23.02.2021 18:10


Chemistry, 23.02.2021 18:10

Biology, 23.02.2021 18:10

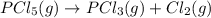
![K_c=\frac{[PCl_3][Cl_2]}{[PCl_5]}](/tpl/images/0439/2265/73fe0.png)
![[PCl_5]](/tpl/images/0439/2265/29487.png) = 0.0095 M
= 0.0095 M ![[PCl_3]](/tpl/images/0439/2265/5e6c7.png) = 0.020 M
= 0.020 M![[Cl_2]](/tpl/images/0439/2265/53f7d.png) = 0.020 M
= 0.020 M ![K_c=\frac{[0.020][0.020]}{[0.0095]}=0.042](/tpl/images/0439/2265/3712e.png)


