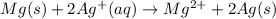An electrochemical cell has the following standard cell notation.
mg(s) | mg^2+ (aq) ||...

Chemistry, 11.10.2019 19:40 abelxoconda
An electrochemical cell has the following standard cell notation.
mg(s) | mg^2+ (aq) || aq^+(aq) | aq(s)
write the balanced oxidation and reduction half reactions, labeling the oxidation reaction and the reduction reaction.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2

Chemistry, 22.06.2019 22:00
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1

Chemistry, 23.06.2019 08:00
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1
You know the right answer?
Questions

History, 02.07.2019 05:00


Mathematics, 02.07.2019 05:00

Mathematics, 02.07.2019 05:00

Health, 02.07.2019 05:00

Biology, 02.07.2019 05:00


English, 02.07.2019 05:00



History, 02.07.2019 05:00





Mathematics, 02.07.2019 05:00

Mathematics, 02.07.2019 05:00

Mathematics, 02.07.2019 05:00

Social Studies, 02.07.2019 05:00

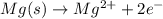 ....(1)
....(1)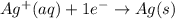 ...(2)
...(2)