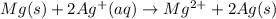An electrochemical cell has the following standard cell notation.
mg(s) | mg^2+ (aq) ||...

An electrochemical cell has the following standard cell notation.
mg(s) | mg^2+ (aq) || aq^+(aq) | aq(s)
write a blanked redox equation for the cell using the oxidation and reduction half reactions. (be sure to equalize charge by multiplying by the correct numbers before adding and simplifying)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2
You know the right answer?
Questions




Social Studies, 08.09.2020 05:01

Social Studies, 08.09.2020 05:01

English, 08.09.2020 05:01

Mathematics, 08.09.2020 05:01

Mathematics, 08.09.2020 05:01





Physics, 08.09.2020 05:01




Arts, 08.09.2020 05:01



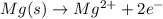 ....(1)
....(1)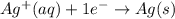 ...(2)
...(2)