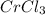The chemical equation for a reaction between k2cr2o7 and hcl is shown. k2cr2o7 + 14hcl → 2crcl3 + 2kcl + 3cl2 + 7h2o
which of the following identifies the reactant that acts as an oxidizing agent in the reaction and explains the answer? 20 !
a. k2cr2o7, because the oxidation number of k changes from +6 to +3.
b. k2cr2o7, because the oxidation number of cr changes from +6 to +3.
c. hcl, because the oxidation number of h changes from −1 to 0.
d. hcl, because the oxidation number of cl changes from −1 to 0.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 20:00
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1

Chemistry, 23.06.2019 16:00
Which is the best metal to use in an alloy to increase its electrical conductivity?
Answers: 2
You know the right answer?
The chemical equation for a reaction between k2cr2o7 and hcl is shown. k2cr2o7 + 14hcl → 2crcl3 + 2k...
Questions


Mathematics, 02.10.2021 06:50


Social Studies, 02.10.2021 06:50


Mathematics, 02.10.2021 06:50


Mathematics, 02.10.2021 06:50

Business, 02.10.2021 06:50





Mathematics, 02.10.2021 06:50



Chemistry, 02.10.2021 07:00

Biology, 02.10.2021 07:00

English, 02.10.2021 07:00

Mathematics, 02.10.2021 07:00

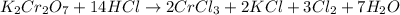
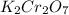 acts like and oxidizing agent because it is itself getting reduced to
acts like and oxidizing agent because it is itself getting reduced to