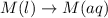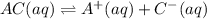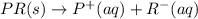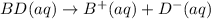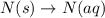Chemistry, 03.02.2020 05:47 mathman783
Each of the following reactions shows a solute dissolved in water. classify each solute as a strong electrolyte, a weak electrolyte, or a nonelectrolyte?
m(l)→m(aq)
ac(aq)⇌a+(aq)+c−(aq)
bd(s)→b+(aq)+d−(aq)
pr(aq)→p+(aq)+r−(aq)
n(s)→n(aq)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:20
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1

Chemistry, 22.06.2019 01:30
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3
You know the right answer?
Each of the following reactions shows a solute dissolved in water. classify each solute as a strong...
Questions

Mathematics, 19.11.2020 19:20

Mathematics, 19.11.2020 19:20




History, 19.11.2020 19:20

Mathematics, 19.11.2020 19:20


Mathematics, 19.11.2020 19:20

Social Studies, 19.11.2020 19:20

Mathematics, 19.11.2020 19:20


Mathematics, 19.11.2020 19:20


Chemistry, 19.11.2020 19:20

Arts, 19.11.2020 19:20

English, 19.11.2020 19:20


Mathematics, 19.11.2020 19:20