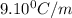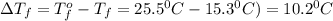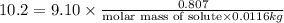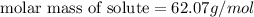Chemistry, 04.02.2020 20:46 Envious1552
Tert-butyl alcohol is a solvent with a kf of 9.10 ∘c/m and a freezing point of 25.5 ∘c. when 0.807 g of an unknown colorless liquid was dissolved in 11.6 g of tert-butyl alcohol, the solution froze at 15.3 ∘c. which of the following is most likely the identity of this unknown liquid? tert-butyl alcohol is a solvent with a of 9.10 and a freezing point of 25.5 . when 0.807 of an unknown colorless liquid was dissolved in 11.6 of tert-butyl alcohol, the solution froze at 15.3 .which of the following is most likely the identity of this unknown liquid? ethylene glycol (molar mass = 62.07 g/mol)1-octanol (molar mass = 130.22 g/mol)glycerol (molar mass = 92.09 g/mol)2-pentanone (molar mass = 86.13 g/mol)1-butanol (molar mass = 74.12 g/mol)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:50
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

You know the right answer?
Tert-butyl alcohol is a solvent with a kf of 9.10 ∘c/m and a freezing point of 25.5 ∘c. when 0.807 g...
Questions

History, 12.07.2019 00:00



Mathematics, 12.07.2019 00:00


Social Studies, 12.07.2019 00:00

English, 12.07.2019 00:00







History, 12.07.2019 00:00






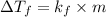
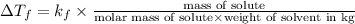
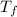 = change in freezing point
= change in freezing point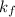 = freezing point constant=
= freezing point constant=