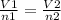Chemistry, 28.01.2020 09:31 granthazenp5e9mj
Gas laws describe and predict the behavior of gases without attempting to explain why they happen
true
false
a 6.0 l sample of nitrogen gas contains 0.50 mole of a gas. if enough gas is added to make a total of 0.75 moles at the same pressure and temperature, what is the resulting total volume of the gas? (4 points)
2.3 l
4.8 l
7.5 l
9.0 l

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2


You know the right answer?
Gas laws describe and predict the behavior of gases without attempting to explain why they happen
Questions






Mathematics, 07.12.2020 21:00



Health, 07.12.2020 21:00



Biology, 07.12.2020 21:00

Mathematics, 07.12.2020 21:00

Mathematics, 07.12.2020 21:00



Mathematics, 07.12.2020 21:00

Mathematics, 07.12.2020 21:00

Geography, 07.12.2020 21:00

Mathematics, 07.12.2020 21:00