
Chemistry, 05.02.2020 04:54 rileyallen4186pd5tgy
Consider the balanced chemical reaction below. when the reaction was carried out, the calculated theoretical yield for carbon dioxide was 93.7 grams, but the measured yield was 88.3 grams. what is the percent yield?
fe2o3 + 3co --> 2fe + 3co2

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
1.aluminum chloride (alcl3), and sodium hydroxide (naoh) can react to form aluminum hydroxide (al(oh)3) and sodium chloride (nacl). you have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide. answer the following questions: •what is the balanced equation for this reaction? •if you use all 13.4 g of aluminum chloride, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •if you use all 10.0 g of sodium hydroxide, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •how many grams of aluminum hydroxide will actually be made? which reagent is limiting? explain your answer.
Answers: 1


Chemistry, 23.06.2019 07:30
Which of the following statements best explains why chemistry is testable a) it can measure data by experiments b) it cannot add new evidence c) it cannot be verified d) it is biased
Answers: 1

Chemistry, 23.06.2019 11:00
The decimals you found in part b are called repeating decimals. in the gizmo, repeating decimals are rounded to two places. how does the gizmo show you that a decimal has been rounded?
Answers: 3
You know the right answer?
Consider the balanced chemical reaction below. when the reaction was carried out, the calculated the...
Questions



Arts, 27.03.2021 23:10


Biology, 27.03.2021 23:10

Social Studies, 27.03.2021 23:10


Arts, 27.03.2021 23:10



Mathematics, 27.03.2021 23:10





Mathematics, 27.03.2021 23:10

Mathematics, 27.03.2021 23:10


Social Studies, 27.03.2021 23:10

English, 27.03.2021 23:10

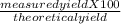
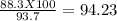 %
%

