Chemistry, 21.10.2019 17:30 gabrielolivas59
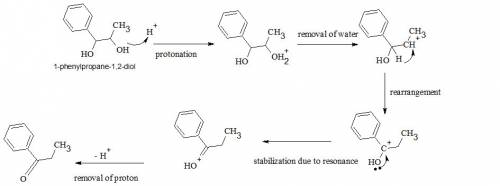
When warmed in dilute sulfuric acid, 1-phenyl-1,2-propanediol undergoes dehydration and rearrangement to give 2-phenylpropanal. its constitutional isomer, 1-phenyl-1-propanone, is not formed under these conditions. following are the mechanistic steps leading to the formation of this unobserved isomer: add a proton to give protonated alcohol 1; break a bond to give carbocation intermediate 2; 1,2-shift to give carbocation intermediate 3; carbocation intermediate 3 is stabilized by resonance delocalization with carbonyl 4; loss of a proton leads to the formation of 1-phenyl-1-propanone. write out the mechanism on a separate sheet of paper, and then draw the structure of carbocation intermediate 3 in the box below. you do not have to consider stereochemistry. you do not have to explicitly draw h atoms. do not include lone pairs in your answer. they will not be considered in the grading.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
You know the right answer?
When warmed in dilute sulfuric acid, 1-phenyl-1,2-propanediol undergoes dehydration and rearrangemen...
Questions






Mathematics, 05.05.2021 02:00


Chemistry, 05.05.2021 02:00

Mathematics, 05.05.2021 02:00

History, 05.05.2021 02:00


English, 05.05.2021 02:00


Mathematics, 05.05.2021 02:00





Mathematics, 05.05.2021 02:00