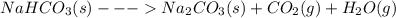Chemistry, 20.01.2020 11:31 kenzietaylre
Sodium carbonate, na2co3(s), can be prepared by heating sodium bicarbonate, nahco3(s). 2nahco3(s) > na2co3(s) + co2(g) + h2o(g) kp = 0.23 at 100ºc if a sample of nahco3 is placed in an evacuated flask and allowed to achieve equilibrium at 100ºc, what will the total gas pressure be? 0.46 atm 0.96 atm 0.23 atm 0.48 atm 0.11 atm

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 23.06.2019 01:00
If a sample of radioactive isotopes takes 600 minutes to decay from 400 grams to 50 grams, what is the half-life of the isotope?
Answers: 1

Chemistry, 23.06.2019 05:50
Aseismic wave is energy released as the result of rock movement along a fault. t or f ?
Answers: 1

You know the right answer?
Sodium carbonate, na2co3(s), can be prepared by heating sodium bicarbonate, nahco3(s). 2nahco3(s) &g...
Questions