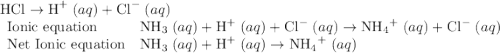Chemistry, 18.12.2019 10:31 lezapancakes13
Which of the chemical equations below are acid-base (proton transfer) reactions? select all that apply. mg(oh)2 (s) + h2so4 (aq) → mgso4 (s) + 2 h2o (l) fe(no3)3 (aq) + 3 koh (aq) → fe(oh)3 (s) + 3 kno3 (aq) zn (s) + cu(no3)2 (aq) → zn(no3)2 (aq) + cu (s) mg (s) + cu(no3)2 (aq) --> mg(no3)3 (aq) + cu (s) hcl (aq) + koh (aq) → kcl (aq) + h2o (l) nh3 (aq) + hcl (aq) → nh4cl (aq)?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 02:00
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
Which of the chemical equations below are acid-base (proton transfer) reactions? select all that ap...
Questions

Computers and Technology, 11.09.2019 19:30









Mathematics, 11.09.2019 19:30


History, 11.09.2019 19:30


Computers and Technology, 11.09.2019 19:30






Computers and Technology, 11.09.2019 19:30