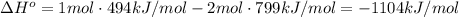Chemistry, 31.10.2019 11:31 24hudsonmoss
Given: c + o2 → co2 bond bond energy (kj/mol) c=o 799 o=o 494 calculate the enthalpy change for the chemical reaction. the change in enthalpy for the given reaction is kilojoules.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2

Chemistry, 23.06.2019 02:00
When an experimenter draws a conclusion that he assumes will apply to all situations set up similarly to his test situation, even though he cannot possibly have examined all possible test scenarios, the experimenter is using deductive reasoning inductive reasoning abductive reasoning subjective reasoning
Answers: 1
You know the right answer?
Given: c + o2 → co2 bond bond energy (kj/mol) c=o 799 o=o 494 calculate the enthalpy change for the...
Questions




Mathematics, 12.09.2019 00:30


Mathematics, 12.09.2019 00:30

Mathematics, 12.09.2019 00:30



Mathematics, 12.09.2019 00:30


Mathematics, 12.09.2019 00:30

Biology, 12.09.2019 00:30



Mathematics, 12.09.2019 00:30

Mathematics, 12.09.2019 00:30


Biology, 12.09.2019 00:30

Computers and Technology, 12.09.2019 00:30