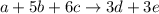Chemistry, 04.11.2019 21:31 dalejacksoniip5yf4y
The balanced equation for a hypothetical reaction is a + 5b + 6c → 3d + 3e. what is the rate law for this reaction? rate = k [a][b]5[c]6 rate = k [a][b][c] rate = k [d]3[e]3

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 22.06.2019 19:30
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2
You know the right answer?
The balanced equation for a hypothetical reaction is a + 5b + 6c → 3d + 3e. what is the rate law for...
Questions











English, 06.11.2019 00:31



German, 06.11.2019 00:31



Mathematics, 06.11.2019 00:31

History, 06.11.2019 00:31


![Rate=k[a][b]^5[c]^6](/tpl/images/0359/2871/fe35d.png)