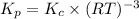Chemistry, 04.02.2020 04:43 kris22elizondop9v1bb
Consider the following equilibrium:
4fe(s) + 3 o2(g) < -- --> 2fe2o3(s);
which of the following equations is wrong?
kp=kc(rt)-5
kc=[o2]-3
kp= kc(rt)-3
kp = po2-3

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
If the particles in a sample of matter have an orderly arrangement and move only in place, the sample is a
Answers: 1

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 14:00
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3
You know the right answer?
Consider the following equilibrium:
4fe(s) + 3 o2(g) < -- --> 2fe2o3(s);
<...
4fe(s) + 3 o2(g) < -- --> 2fe2o3(s);
<...
Questions


Mathematics, 16.02.2021 22:00

Chemistry, 16.02.2021 22:00


Mathematics, 16.02.2021 22:00

Mathematics, 16.02.2021 22:00





Computers and Technology, 16.02.2021 22:00



History, 16.02.2021 22:00




History, 16.02.2021 22:00

Mathematics, 16.02.2021 22:00

Arts, 16.02.2021 22:00

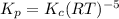
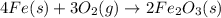
 is given by:
is given by:![K_c=\frac{1}{[O_2]^3}](/tpl/images/0499/0672/4f5e0.png)
![K_p=\frac{1}{[O_2]^3}](/tpl/images/0499/0672/d3f6c.png)
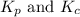 is given by the expression:
is given by the expression: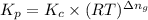
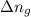 = number of moles of gaseous products - number of moles of gaseous reactants
= number of moles of gaseous products - number of moles of gaseous reactants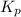 is:
is: