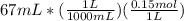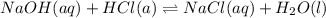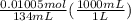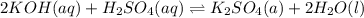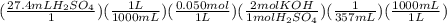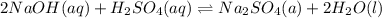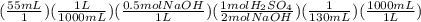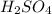Chemistry, 20.01.2020 13:31 skpdancer1605
1if it takes 67 ml of 0.15 m naoh to neutralize 134 ml of an hcl solution, what is the concentration of the hcl? (5 points)
2 if it takes 27.4 ml of 0.050 m h2so4 to neutralize 357 ml of koh solution, what is the concentration of the naoh solution? (5 points)
3 if it takes 55 ml of 0.5 m naoh solution to completely neutralize 130 ml of sulfuric acid solution (h2so4), what is the concentration of the h2so4 solution? (5 points)
4 explain the difference between an endpoint and equivalence point in a titration. (5 points)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1
You know the right answer?
1if it takes 67 ml of 0.15 m naoh to neutralize 134 ml of an hcl solution, what is the concentration...
Questions

Mathematics, 14.02.2021 08:50

Mathematics, 14.02.2021 08:50


Mathematics, 14.02.2021 08:50

Mathematics, 14.02.2021 09:00



Mathematics, 14.02.2021 09:00




English, 14.02.2021 09:00

Spanish, 14.02.2021 09:00

Health, 14.02.2021 09:00

Arts, 14.02.2021 09:00


Computers and Technology, 14.02.2021 09:00