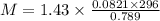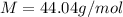Chemistry, 29.01.2020 04:43 kingken3400
The density of a gas is 1.43 g/l at a temperature of 23 ∘c and a pressure of 0.789 atm. calculate the molar mass of the gas.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which sentence best describes the formation of igneous rock? a- lava on the surface dries up and makes arock b_melted rocks cools and forms crystals c_rocks under tremendous heat and pressure d_magma is melted rock underground
Answers: 1

Chemistry, 22.06.2019 00:00
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
You know the right answer?
The density of a gas is 1.43 g/l at a temperature of 23 ∘c and a pressure of 0.789 atm. calculate th...
Questions



Health, 04.02.2020 07:55

Biology, 04.02.2020 07:55

Mathematics, 04.02.2020 07:55

Mathematics, 04.02.2020 07:55

Arts, 04.02.2020 07:55


Social Studies, 04.02.2020 07:55

Business, 04.02.2020 07:55

Physics, 04.02.2020 07:55




Mathematics, 04.02.2020 07:56



Mathematics, 04.02.2020 07:56

History, 04.02.2020 07:56

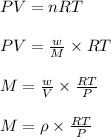
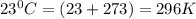
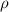 = density =1.43 g/ml
= density =1.43 g/ml