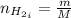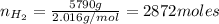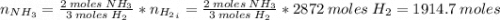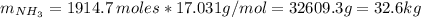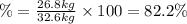Chemistry, 28.09.2019 15:10 victoriacarr638
Areaction vessel for synthesizing ammonia by reacting nitrogen and hydrogen is charged with 5.79 kg of h2 and excess n2. a total of 26.8 kg of nh3 are produced. what is the percent yield of the reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1
You know the right answer?
Areaction vessel for synthesizing ammonia by reacting nitrogen and hydrogen is charged with 5.79 kg...
Questions

History, 24.02.2020 19:51








Mathematics, 24.02.2020 19:51


Biology, 24.02.2020 19:51


Physics, 24.02.2020 19:51







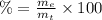 (2)
(2)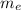 : is the experimental (or actual) mass = 26.8 kg
: is the experimental (or actual) mass = 26.8 kg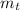 : is the theoretical mass
: is the theoretical mass