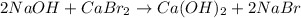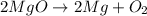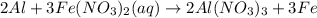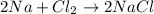Chemistry, 24.09.2019 13:30 sophiav9780
Given the following reactants, determine the type of reaction that will take place, predict the products ,and produce a balanced chemical equation
sodium hydroxide and calcium bromide
magnesium oxide
aluminum and iron (ii) nitrate
sodium and chlorine

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2
You know the right answer?
Given the following reactants, determine the type of reaction that will take place, predict the prod...
Questions

Biology, 30.03.2021 22:10





English, 30.03.2021 22:10

SAT, 30.03.2021 22:10

Mathematics, 30.03.2021 22:10


Arts, 30.03.2021 22:10



Mathematics, 30.03.2021 22:10

Mathematics, 30.03.2021 22:10



Social Studies, 30.03.2021 22:10


Mathematics, 30.03.2021 22:10