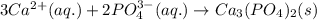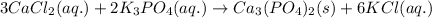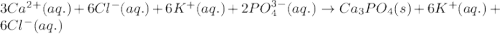Chemistry, 29.01.2020 19:53 genyjoannerubiera
Predict what precipitate will be formed using the solubility rules. then write the net ionic equation for the formation of the solid. balance the net equation.
cacl2(aq) + k3po4(aq)
i’ve already got a majority of the net ionic equation, capo4(aq) + cl2k3(aq) = ?
but i don’t know what the solid will end up being

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:20
Which type of substance ionizes partially and gives off hydrogen ions when dissolved in water? a. strong acid b. strong base c. weak acid d. weak base
Answers: 1

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1
You know the right answer?
Predict what precipitate will be formed using the solubility rules. then write the net ionic equatio...
Questions

Business, 11.11.2021 09:10

History, 11.11.2021 09:10

Mathematics, 11.11.2021 09:10



Chemistry, 11.11.2021 09:10

Social Studies, 11.11.2021 09:10

Chemistry, 11.11.2021 09:10

Mathematics, 11.11.2021 09:10



Biology, 11.11.2021 09:20

Mathematics, 11.11.2021 09:20

Mathematics, 11.11.2021 09:20


Mathematics, 11.11.2021 09:20




Mathematics, 11.11.2021 09:20