
Chemistry, 30.01.2020 07:50 wrerteteT2827
Consider the following reaction at equilibrium: 2co2 (g) ↔ 2co (g) + o2 (g) ∆h° = -514 kj le châtelier's principle predicts that removing o2(g) to the reaction container will consider the following reaction at equilibrium: 2co2 (g) 2co (g) + o2 (g) ∆h° = -514 kj le châtelier's principle predicts that removing o2(g) to the reaction container will decrease the value of the equilibrium constant increase the partial pressure of co increase the value of the equilibrium constant decrease the partial pressure of co increase the partial pressure of co2

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1

Chemistry, 22.06.2019 01:00
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3
You know the right answer?
Consider the following reaction at equilibrium: 2co2 (g) ↔ 2co (g) + o2 (g) ∆h° = -514 kj le châtel...
Questions






Advanced Placement (AP), 09.09.2020 19:01

Health, 09.09.2020 19:01



Mathematics, 09.09.2020 19:01


Mathematics, 09.09.2020 19:01


Mathematics, 09.09.2020 19:01



History, 09.09.2020 19:01



Mathematics, 09.09.2020 19:01

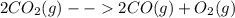 ∆H° = -514
∆H° = -514 

