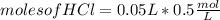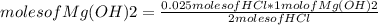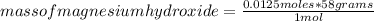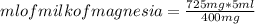Chemistry, 27.09.2019 11:20 bravomichelle75
For every 5.00 ml of milk of magnesia there are 400. mg of magnesium hydroxide. how many ml of milk of magnesia do we need to neutralize? for every 5.00 ml of milk of magnesia there are 400. mg of magnesium hydroxide. how many ml of milk of magnesia do we need to neutralize 50.0 ml of 0.500 m hcl? express your answer in ml.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Read these sentences from the text. near the equator, the tropics receive the most rain on a consistent basis. as a result, the fresh water falling into the ocean decrease the salinity of the surface water in that region. [. .] . . as the salt content of sea water increases, so does its density. what can you infer about how rain affects the density of surface water near the equator?
Answers: 1

Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1

Chemistry, 23.06.2019 01:00
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1

Chemistry, 23.06.2019 09:50
T(s) in2os] (m) 0 185 2.39 546 1.90 725 1.70 the decomposition of n205 can be described by the equation 2.68 given these data for the reaction at 45°c in carbon tetrachloride solution, calculate the average rate of reaction for each successive time interval. ntr s to 185 s 185 s to 546 s 546 s to 725 s number number number reaction rate: m/s m/s m/s
Answers: 1
You know the right answer?
For every 5.00 ml of milk of magnesia there are 400. mg of magnesium hydroxide. how many ml of milk...
Questions

History, 27.03.2020 23:20




Mathematics, 27.03.2020 23:20

History, 27.03.2020 23:20

Mathematics, 27.03.2020 23:20


English, 27.03.2020 23:20

Mathematics, 27.03.2020 23:20

Biology, 27.03.2020 23:20

Arts, 27.03.2020 23:20


English, 27.03.2020 23:20


English, 27.03.2020 23:21


Mathematics, 27.03.2020 23:21

Mathematics, 27.03.2020 23:21