Chemistry, 05.02.2020 09:53 lanipooh01
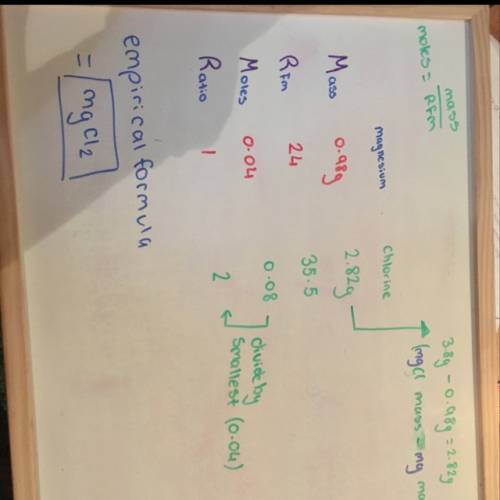
0.98 g of magnesium combined with chlorine gas to form 3.8 g of magnesium chloride. determine the empirical formula of the product.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no2]2 ? second order 3/2 order third order zero order none of the listed answers are correct
Answers: 3

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1

Chemistry, 23.06.2019 03:00
The size (radius) of an oxygen molecule is about 2.0 ×10−10m. make a rough estimate of the pressure at which the finite volume of the molecules should cause noticeable deviations from ideal-gas behavior at ordinary temperatures (t= 300k ). assume that deviatons would be noticeable when volume of the gas per molecule equals the volume of the molecule itself.
Answers: 3

You know the right answer?
0.98 g of magnesium combined with chlorine gas to form 3.8 g of magnesium chloride. determine the em...
Questions


Mathematics, 02.07.2020 19:01

Mathematics, 02.07.2020 19:01

Mathematics, 02.07.2020 19:01

History, 02.07.2020 19:01

Law, 02.07.2020 19:01

Health, 02.07.2020 19:01

Mathematics, 02.07.2020 19:01



Biology, 02.07.2020 19:01



Computers and Technology, 02.07.2020 19:01



Mathematics, 02.07.2020 19:01


Mathematics, 02.07.2020 19:01

Mathematics, 02.07.2020 19:01