
Chemistry, 21.11.2019 09:31 rubianny03
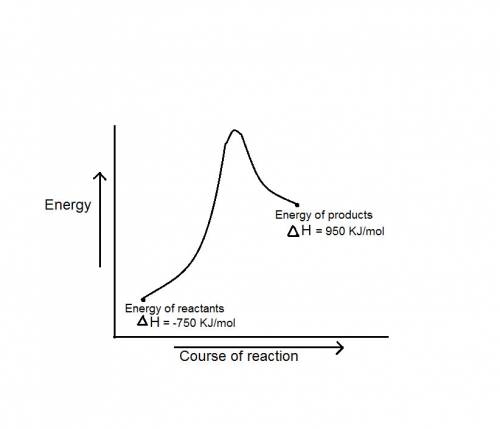
during a reaction, δh for reactants is −750 kj/mol and δh for products is 920 kj/mol. which statement is correct about the reaction?
it is endothermic because the energy required to break bonds in the reactants is less than the energy released when the products are formed.
it is endothermic because the energy required to break bonds in the reactants is greater than the energy released when the products are formed.
it is exothermic because the energy required to break bonds in the reactants is less than the energy released when the products are formed.
it is exothermic because the energy required to break bonds in the reactants is greater than the energy released when the products are formed.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
10-14. (a) when 100.0 ml of weak acid ha were titrated with 0.093 81 m naoh, 27.63 ml were required to reach the equivalence point. find the molarity of ha. (b) what is the formal concentration of a- at the equivalence point? (c) the ph at the equivalence point was 10.99. find pk. for ha. (d) what was the ph when only 19.47 ml of naoh had been added?
Answers: 1

Chemistry, 22.06.2019 23:00
What extra step distinguishes fermentation from glycolysis
Answers: 1

Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1

Chemistry, 23.06.2019 02:00
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1
You know the right answer?
during a reaction, δh for reactants is −750 kj/mol and δh for products is 920 kj/mol. which statemen...
Questions







Computers and Technology, 20.09.2020 16:01

Mathematics, 20.09.2020 16:01

Chemistry, 20.09.2020 16:01




Mathematics, 20.09.2020 16:01

History, 20.09.2020 16:01

Business, 20.09.2020 16:01

Computers and Technology, 20.09.2020 16:01


Mathematics, 20.09.2020 16:01

English, 20.09.2020 16:01

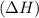 comes out to be positive.
comes out to be positive.

