
Chemistry, 14.12.2019 04:31 araminaara691
The freezing of methane is an exothermic change. what best describes the temperature conditions that are likely to make this a spontaneous change? any temperature, because entropy increases during freezing. any temperature, because entropy decreases during freezing. low temperature only, because entropy decreases during freezing. high temperature only, because entropy increases during freezing.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:00
2h2s + 3o2 2so2 + 2h2o which option gives the correct mole ratios? h2s: so2 = 2: 2 and o2: h2o = 3: 2 h2s: so2 = 2: 3 and o2: h2o = 3: 2 h2s: so2 = 4: 4 and o2: h2o = 5: 4 h2s: so2 = 4: 6 and o2: h2o = 4: 4
Answers: 1

Chemistry, 22.06.2019 01:00
According to the tide table below what time of day will the highest tide occur?
Answers: 1

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2

Chemistry, 22.06.2019 21:30
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1
You know the right answer?
The freezing of methane is an exothermic change. what best describes the temperature conditions that...
Questions


Mathematics, 02.03.2021 03:20



Mathematics, 02.03.2021 03:20




History, 02.03.2021 03:20




English, 02.03.2021 03:20

History, 02.03.2021 03:20




Mathematics, 02.03.2021 03:20


Mathematics, 02.03.2021 03:20

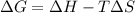
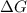 = change in Gibb's free energy
= change in Gibb's free energy 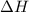 = change in enthalpy
= change in enthalpy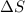 = change in entropy
= change in entropy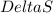 is decreasing and its sign is negative. This reaction is an exothermic reaction, which means that the
is decreasing and its sign is negative. This reaction is an exothermic reaction, which means that the ![-ve=-ve-[T(-ve)]\\\\-ve=-ve+T](/tpl/images/0418/2588/a4550.png)
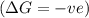 , the temperature must be low.
, the temperature must be low.

