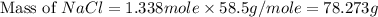Chemistry, 31.01.2020 23:04 sjjarvis53211
Part 1. a chemist reacted 15.0 liters of f2 gas with nacl in the laboratory to form cl2 and naf. use the ideal gas law equation to determine the mass of nacl that reacted with f2 at 280. k and 1.50 atm.
f2 + 2nacl → cl2 + 2naf
part 2. explain how you would determine the mass of sodium chloride that can react with the same volume of fluorine gas at stp.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3
You know the right answer?
Part 1. a chemist reacted 15.0 liters of f2 gas with nacl in the laboratory to form cl2 and naf. use...
Questions


Social Studies, 29.10.2019 18:31



Chemistry, 29.10.2019 18:31

Mathematics, 29.10.2019 18:31

History, 29.10.2019 18:31

History, 29.10.2019 18:31

Computers and Technology, 29.10.2019 18:31


Mathematics, 29.10.2019 18:31

Advanced Placement (AP), 29.10.2019 18:31


Chemistry, 29.10.2019 18:31

Mathematics, 29.10.2019 18:31




Mathematics, 29.10.2019 18:31

Mathematics, 29.10.2019 18:31

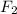 gas by using ideal gas equation.
gas by using ideal gas equation.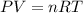
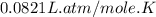
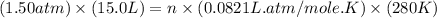
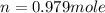
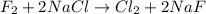
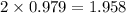 moles of NaCl
moles of NaCl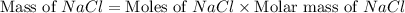
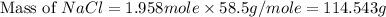
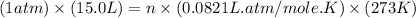
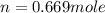
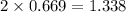 moles of NaCl
moles of NaCl