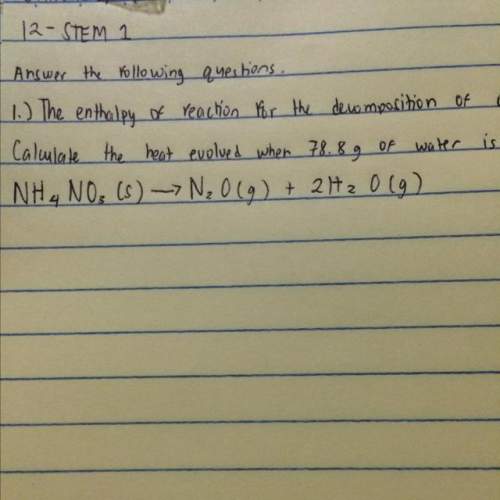Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Amass of 100.0 g of solute is dissolved in water so that 850. ml of a 0.7500 m solution has been prepared. what is the molar mass of the solute?
Answers: 2


Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1
You know the right answer?
The enthalpy of reaction for the decomposition of ammonium nitrate, nh4no3 is -77.4 kj mol^-1. calcu...
Questions


Mathematics, 17.03.2021 23:50

Physics, 17.03.2021 23:50


Mathematics, 17.03.2021 23:50

English, 17.03.2021 23:50

Mathematics, 17.03.2021 23:50

Mathematics, 17.03.2021 23:50



Mathematics, 17.03.2021 23:50


Mathematics, 17.03.2021 23:50

Mathematics, 17.03.2021 23:50

Arts, 17.03.2021 23:50



Mathematics, 17.03.2021 23:50


Chemistry, 17.03.2021 23:50