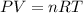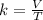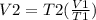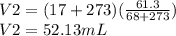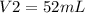Chemistry, 02.10.2019 22:30 emmalouh5986
Acertain quantity of a gas occupies 61.3 ml at 68°c. if the pressure remains constant, what would the volume of the gas be at 17°c? a. 52 mlb. 72 mlc. 32 mld. 92 ml

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3


Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2
You know the right answer?
Acertain quantity of a gas occupies 61.3 ml at 68°c. if the pressure remains constant, what would th...
Questions


Mathematics, 13.10.2020 15:01




History, 13.10.2020 15:01

Mathematics, 13.10.2020 15:01


Computers and Technology, 13.10.2020 15:01





English, 13.10.2020 15:01

Biology, 13.10.2020 15:01


Engineering, 13.10.2020 15:01

Mathematics, 13.10.2020 15:01