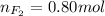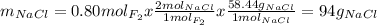Part 1. a chemist reacted 18.0 liters of f2 gas with nacl in the laboratory to form cl2 gas and naf. use the ideal gas law equation to determine the mass of nacl that reacted with f2 at 290. k and 1.5 atm.
f2 + 2nacl → cl2 + 2naf
part 2. explain how you would determine the mass of sodium chloride that can react with the same volume of fluorine gas at stp.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1


Chemistry, 22.06.2019 23:30
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd a) 30 joules b) 3000 joules c) 320 joules d) 3200 joules
Answers: 2
You know the right answer?
Part 1. a chemist reacted 18.0 liters of f2 gas with nacl in the laboratory to form cl2 gas and naf....
Questions


English, 07.03.2021 01:10


Mathematics, 07.03.2021 01:10






Mathematics, 07.03.2021 01:10


Health, 07.03.2021 01:10

Social Studies, 07.03.2021 01:10

Mathematics, 07.03.2021 01:10



English, 07.03.2021 01:10

Biology, 07.03.2021 01:10

Social Studies, 07.03.2021 01:10

Mathematics, 07.03.2021 01:10

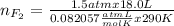 →
→ 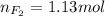
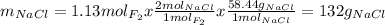
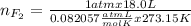 →
→