
Chemistry, 06.10.2019 07:11 kberly3750ovgw6f
According to the following balanced equation, 2 formula units of iron (iii) oxide (fe2o3) can be formed by reacting 4 atoms of iron (fe) with 3 molecules of oxygen gas (o2). if 12 atoms of iron are reacted with 6 molecules of oxygen gas, which is the limiting reactant and how many atoms or molecules will be left over? 4fe + 3o2 -> 2fe2o3

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1

Chemistry, 22.06.2019 03:00
Explain how the integumentary system plays a crucial role in the ability to maintain homeoestasis
Answers: 1

Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1
You know the right answer?
According to the following balanced equation, 2 formula units of iron (iii) oxide (fe2o3) can be for...
Questions

History, 19.11.2020 19:40

Chemistry, 19.11.2020 19:40






Chemistry, 19.11.2020 19:40

Arts, 19.11.2020 19:40


Chemistry, 19.11.2020 19:40



Mathematics, 19.11.2020 19:40


History, 19.11.2020 19:40

Chemistry, 19.11.2020 19:40

Mathematics, 19.11.2020 19:40

Mathematics, 19.11.2020 19:40

Mathematics, 19.11.2020 19:40

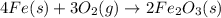
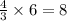 atoms of iron.
atoms of iron.

