
Chemistry, 07.12.2019 17:31 xXCoryxKenshinXx
Calculate the mass of chromium metal produced when 425.0ml of 0.25m chromium(ll) nitrate reacts with a strip of zinc that remains in excess. also write and balance an equation.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Asmall amount of a solid is added to water. the observation made after fifteen minutes is shown in the figure. which of these solids has been probably added to water? a) oil b) sand c) sugar d) wood chips
Answers: 1


Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1
You know the right answer?
Calculate the mass of chromium metal produced when 425.0ml of 0.25m chromium(ll) nitrate reacts with...
Questions

Mathematics, 17.09.2020 04:01

Mathematics, 17.09.2020 04:01

Mathematics, 17.09.2020 04:01

Chemistry, 17.09.2020 04:01

Mathematics, 17.09.2020 04:01

Mathematics, 17.09.2020 04:01

Mathematics, 17.09.2020 04:01

Mathematics, 17.09.2020 04:01

Mathematics, 17.09.2020 04:01

Mathematics, 17.09.2020 04:01

Mathematics, 17.09.2020 04:01

Mathematics, 17.09.2020 04:01

Mathematics, 17.09.2020 04:01

German, 17.09.2020 04:01

Mathematics, 17.09.2020 04:01

Mathematics, 17.09.2020 04:01

Mathematics, 17.09.2020 04:01

Mathematics, 17.09.2020 04:01

English, 17.09.2020 04:01

French, 17.09.2020 04:01

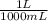 x
x 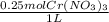 x
x 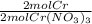 x
x 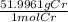 = 5.52 g
= 5.52 g 

