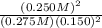Chemistry, 23.12.2019 00:31 McKenzie8409
At a certain temperature the initial concentration of no was 0.400 m and that of br2 was 0.245 m . at equilibrium the concentration of nobr was found to be 0.250 m. what is the value of kc at this temperature? what is the rate of the reaction when the initial concentration of no is 0.400 m and that of br2 is 0.245 m ?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1
You know the right answer?
At a certain temperature the initial concentration of no was 0.400 m and that of br2 was 0.245 m . a...
Questions


Chemistry, 01.07.2020 15:01


Physics, 01.07.2020 15:01


Mathematics, 01.07.2020 15:01






Physics, 01.07.2020 15:01








![\frac{[NOBr]^{2} }{[Br_{2} ] [NO]^{2} }](/tpl/images/0430/1121/7f6be.png)