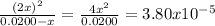The dissociation of molecular iodine into iodine atoms is represented as i2(g) ⇌ 2i(g) at 1000 k, the equilibrium constant kc for the reaction is 3.80 × 10−5. suppose you start with 0.0456 mol of i2 in a 2.28−l flask at 1000 k. what are the concentrations of the gases at equilibrium? what is the equilibrium concentration of i2

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:00
The density of a planet is 0.69 g/cm3 (density of water is 1.0 g/cm3). which of the following planets might this be? a. mercury b. venus c. saturn d. mars
Answers: 3

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3
You know the right answer?
The dissociation of molecular iodine into iodine atoms is represented as i2(g) ⇌ 2i(g) at 1000 k, th...
Questions

Mathematics, 18.09.2021 06:50

Social Studies, 18.09.2021 06:50

Biology, 18.09.2021 06:50



Mathematics, 18.09.2021 06:50


Mathematics, 18.09.2021 06:50



English, 18.09.2021 06:50

Mathematics, 18.09.2021 06:50





Mathematics, 18.09.2021 06:50


Mathematics, 18.09.2021 06:50

![\frac{[I]^{2} }{[I_{2} ]}](/tpl/images/0472/7611/f811c.png)
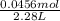 → [I₂]₀ = 0.0200 M
→ [I₂]₀ = 0.0200 M![\frac{[I]^{2} }{[I_{2} ]} = \frac{(2x)^{2} }{0.0200 - x} = 3.80x10^{-5}](/tpl/images/0472/7611/3afcc.png)